Electron transitions in the hydrogen atoms
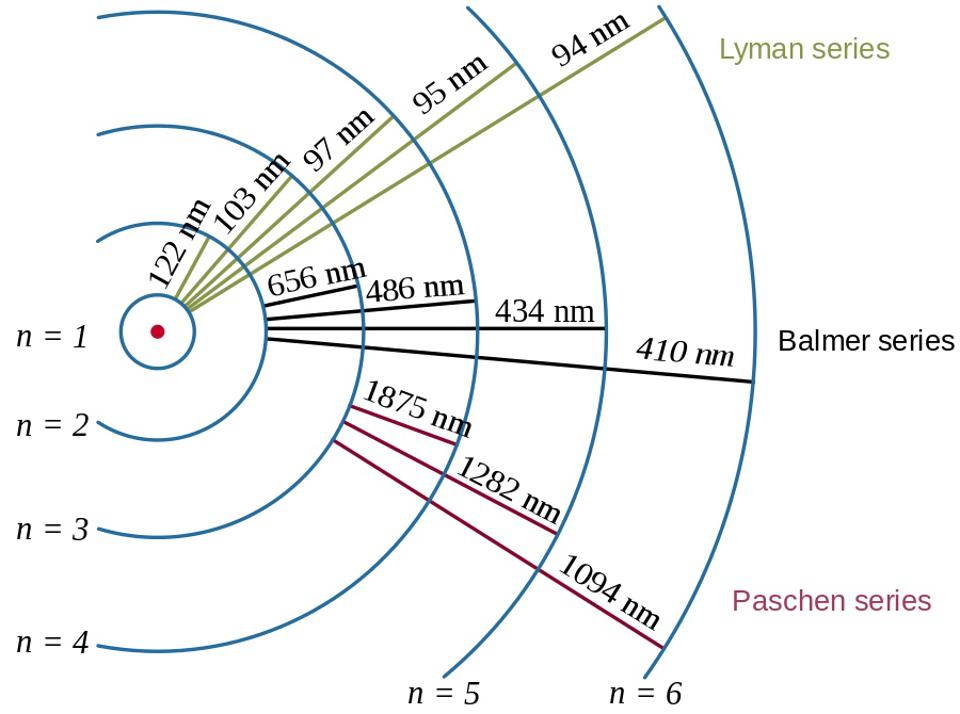
Electron transitions in the hydrogen atom, along with the wavelengths of the resultant photons, showcase the effect of binding energy and the relationship between the electron and the proton in quantum physics.
The Bohr model of the atom provides the coarse (or rough, or gross) structure of these energy levels.
Hydrogen’s brightest atomic transition is Lyman-alpha (n=2 to n=1), but its second brightest is visible: Balmer-alpha (n=3 to n=2), which emits visible (red) light at a wavelength of 656 nanometers. The energy lost by an electron cascading down the energy levels gets emitted in the form of photons.
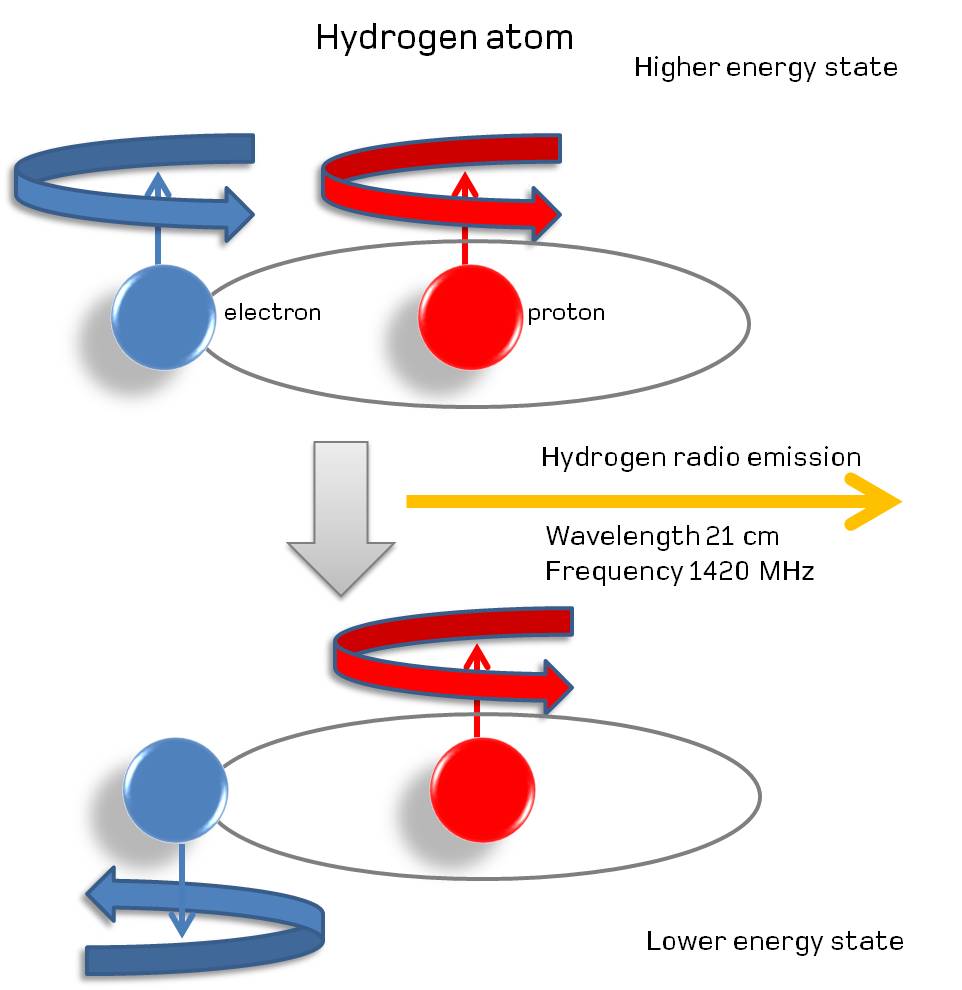
Whenever a neutral hydrogen atom forms, the electron within it will spontaneously de-excite until it’s in the lowest (1s) state of the atom. With a 50/50 chance of having those spins of the electron and proton aligned, half of those atoms will be able to quantum tunnel into the anti-aligned state, emitting radiation of 21 centimeters (1420 MHz) in the process. This should allow us to probe clumps of neutral hydrogen even farther back than the existence of the first stars.
This transition, because of its “forbidden” nature, takes an extremely long time to occur: approximately 10 million years for the average atom. However, this long lifetime of the slightly excited, aligned case for a hydrogen atom has an upside to it: the photon that gets emitted, at 21 centimeters in wavelength and with a frequency of 1420 megahertz, is intrinsically, extremely narrow. In fact, it’s the narrowest, most precise transition line known in all of atomic and nuclear physics!
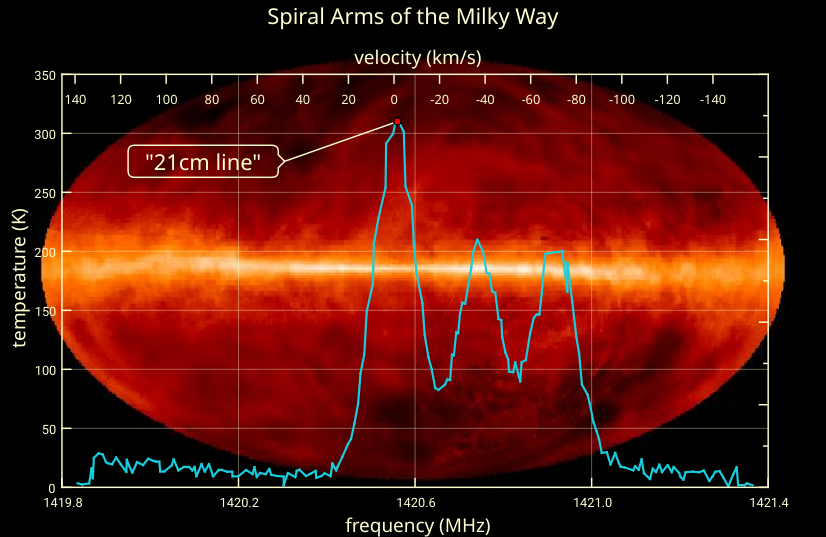
source: https://bigthink.com/starts-with-a-bang/21cm-magic-length/